One of the Earth’s Planetary Boundaries, measured in terms of Aragonite saturation (a measure of carbonate ion concentration.)
Ecological Aspects
Chemistry
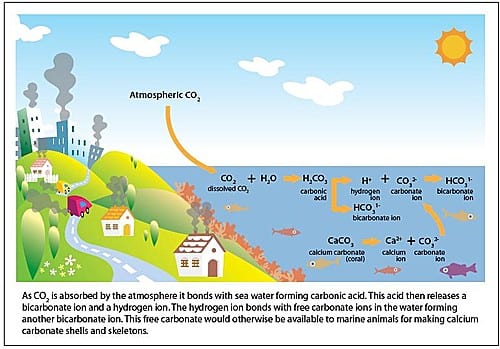
- Part of he carbon dioxide released into the atmosphere gets absorbed by the Oceans
- Carbon absorbed by oceans make the ph of water more acid
Consequences
- Acidification causes faster calcification of marine organisms (coral reef become colorless calcium-looking things)
- Other organisms also do not thrive in acid ocean
Other ocean stressors
- Ocean warming
- Ocean pollution
Connections
- Driven by carbon dioxide (just like climate change
- Chemical pollution comes from food production Food Systems
-model-example.png)
Social Aspects
Governance Difficulties
From Ekstrom, J. A., & Crona, B. I. (2017). Institutional misfit and environmental change: A systems approach to address ocean acidification.
- Perfect cocktail of coordination failures - multipolar traps (Moloch) dilutes incentives for sovereign states to act, individually or in collaboration
- Global sources - Local effects
- Interactions are not well understood
- Interventions would interact with other institutions
While OA is a global concern, effects will be particular- ly discernable along coastlines characterized by upwelling or coral reefs. Such regional ‘OA hotspots’ therefore warrant not just a global, but a multilevel approach to governance. At the international level multilater- al environmental agreements (MEAs) addressing OA are remarkably absent. Some have proposed that OA can be dealt with through UNFCCC, as both climate change and OA share the root cause ofincreas- ing CO2 in the atmosphere (Doney et al., 2009) but others argue that UNFCCC does not provide an adequate legal framework as OA is not an effect of climate change and as such falls outside the UNFCCC’sjuris- diction (Harrould-Kolieb and Herr, 2012; Kim, 2012). This absence of multilateral agreements for policy coordination among states observed for OA has been described as a nonregime (Dimitrov et al., 2007)